



What is liquid crystal? There are three common states of matter that most people know about: solid, liquid, and gas. Liquid crystal is a fourth ``state'' that certain kinds of matter can enter into under the right conditions. The molecules in solids exhibit both positional and orientational order---in other words, the molecules are constrained to point only certain directions and to be only in certain positions with respect to each other. In liquids, the molecules do not have any positional or orientational order---the direction the molecules point and their positions are random.
The liquid crystal ``phase'' exists between the solid and the liquid phase---the molecules in liquid crystal do not exhibit any positional order, but they do possess a certain degree of orientational order. The molecules do not all point the same direction all the time. They merely tend to point more in one direction over time than other directions. This direction is referred to as the director of the liquid crystal. The ``amount'' of order is measured by the order parameter of the liquid crystal, which can be found by averaging the function

for all the
molecules in the sample, where 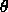 is the angle the long axis of
the molecule makes with a particular direction. Averaging this
function of
is the angle the long axis of
the molecule makes with a particular direction. Averaging this
function of 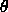 instead of just theta alone conveniently gives a
value between 0 and 1 for the amount of orientational order. This
order parameter is highly dependent on the temperature of the sample.
See Figure 1 for a typical order vs. temperature relationship.
instead of just theta alone conveniently gives a
value between 0 and 1 for the amount of orientational order. This
order parameter is highly dependent on the temperature of the sample.
See Figure 1 for a typical order vs. temperature relationship.
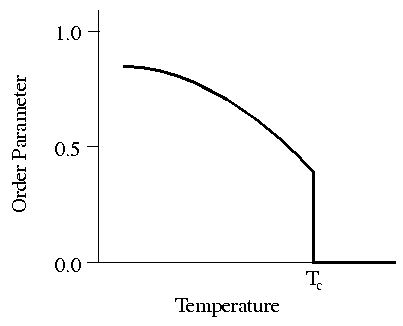
Figure 1: Order
vs. temperature for a typical liquid crystal. Tc is the temperature of
transition between the liquid crystal and liquid states.
Not all substances can have a liquid crystal phase. Molecules that tend to be candidates for having the phase are long and have a rigid central region and ends that are slightly flexible. Liquid crystals are essentially more like liquids than they are like solids. This is evident from the latent heat of transition---the amount of energy needed for a phase transition to occur. In cholesteryl myristate, the latent heat for changing from a solid to the liquid crystal state is 65 calories per gram, whereas the latent heat for the liquid crystal to liquid transition is only 7 calories per gram.
There are many kinds of liquid crystal. Nematic liquid crystal is a common type. The name comes from the Greek word for ``thread'' because of the thread-like formations that defects in this kind of liquid crystal form which can be seen under a microscope. Chiral nematic (or cholesteric) liquid crystal exhibits a twisted structure---literally, the director rotates about an axis as you move through the material. This helical structure is exploited in several ways in making modern flat-panel displays. Smectic liquid crystal has a soapy texture and retains some positional order in that the molecules concentrate in planar layers. Quite often, mixing different kinds of liquid crystal can result in a material that has more useful properties. These lytropic liquid crystal substances are extremely important in display applications.
Liquid crystal has a number of unique characteristics that makes it very suitable for use in displays. Most of these characteristics stem from the fact that liquid crystal is an anisotropic material, meaning that the properties of the material differ depending on what direction they are measured. Liquid crystal behaves differently depending on what direction electric or magnetic fields are applied relative to the director. Since light is an electromagnetic wave, what happens to light as it passes through the liquid crystal will also depend highly on the direction of its propagation and polarization with respect to the director of the crystal.
First, let's consider electric fields. Applying an electric field to a liquid crystal molecule with a permanent electric dipole will cause the dipole to align with the field. If the molecule did not originally have a dipole, then it is induced when the field is applied. Either of these situations has the ultimate effect of aligning the director of the liquid crystal with the electric field being applied. The order of the liquid crystal has not increased---we have only achieved alignment of the director. Only a very weak electric field is needed to accomplish this in liquid crystal. In solids, applying an electric field has little effect because the molecules are held in place by their bonds to other molecules, and in liquids, the high kinetic energy of the molecules makes it impossible to orient them by simply applying an electric field.
Since the electric dipole across liquid crystal molecules varies in degree along the length and the width of the molecule, some kinds of liquid crystal require less electric field and some require much more in order to align the director. The ratio of electric dipole per unit volume of crystal to the field strength is called the electric susceptibility and provides a measure of how easy it is to electrically polarize the material. These ideas are analogous when dealing with magnetic fields. Magnetic dipoles can be inherent, or more likely, they can be induced in the crystal by applying a magnetic field, and there is a corresponding magnetic susceptibility associated with the material.
Liquid crystal is birefringent, meaning that it possesses two different indices of refraction. One index of refraction corresponds to light polarized along the director of the liquid crystal, and the other is for light polarized perpendicular to the director. Light propagating along a certain direction has electric and magnetic field components perpendicular to that direction. Supposing that the director lies perpendicular to the direction of propagation of the light, we can think of each component as being made up of two more components, one parallel to the director of the crystal and one perpendicular. Essentially, what this birefringence property amounts to is that these two components of either the electric or the magnetic field will propagate through the liquid crystal at different speeds and, therefore, possibly be out of phase when they exit the crystal.
If we linearly polarize light before it enters the liquid crystal, meaning that we only allow the electric and magnetic field components of the light to oscillate in one direction, then the light will not be linearly polarized when it leaves the liquid crystal (unless the angle between the polarization direction and the director of the crystal is 0 or 90 degrees). This is because the component of the light perpendicular to the director will ``get ahead'' of the component of light parallel to the director. Depending on how far out of phase the two waves are when they emerge (which depends on the thickness of the crystal), the resulting light might again be linearly polarized, or more likely, the light will be elliptically polarized, meaning that the polarization of the light will rotate around the direction of propagation in some fashion.
Chiral nematic liquid crystal is birefringent in a different way. Supposing that the helical structure is aligned with the direction of propagation of the light, circularly polarized light will travel through the crystal at different speeds depending on whether it is right-circularly polarized or left-circularly polarized (referring to the direction the polarization rotates around the axis of propagation). This is called circular birefringence, and it is exhibited in chiral nematic liquid crystal because of the helical structure the molecules form. The circular birefringence is highly wavelength dependent, so light of different colors gets modified in different amounts.
Circularly polarized light can be thought of containing two components, one of left- and one of right-circularly polarized light. If the light is linearly polarized, these components are equal. If we send linearly polarized light through chiral nematic liquid crystal, the component of circularly polarized light that matches the chirality of the crystal structure will travel faster than the other component, having the ultimate effect that its polarization will rotate faster with respect to the other component's polarization. When the two components emerge, their polarizations will again rotate at the same rate and the light will again be linearly polarized, but since one of the rotations got ahead of the other, the light will now be linearly polarized along a different angle. What the new angle is will depend on how far out of ``circular phase'' the two components were, which is directly dependent on thickness of the liquid crystal. This effect is called the optical activity---it is a measure of the change in polarization angle per unit thickness, and it is heavily wavelength dependent.
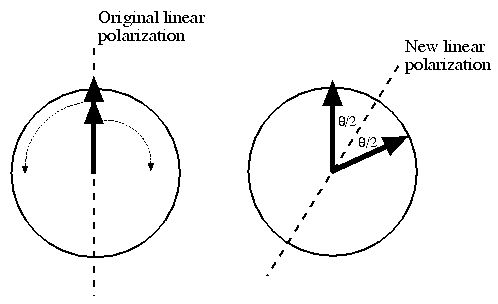
Figure 2: Optical activity. The two arrows represent the
directions of the polarizations of the components of left and
right circularly polarized light at a particular point in
time. After passing through the liquid crystal (right), the
two components sum to create linear polarization in a
different direction.


